Therapy Acceleration Program
Portfolio

Therapy Acceleration Program
Portfolio
Since 2017, four TAP-supported therapies have been approved by the U.S. Food and Drug Administration (FDA) or included in the National Comprehensive Cancer Network (NCCN) Guidelines:
Currently, there are over 30 active clinical studies with TAP-supported assets,
including several registration-enabling clinical studies.
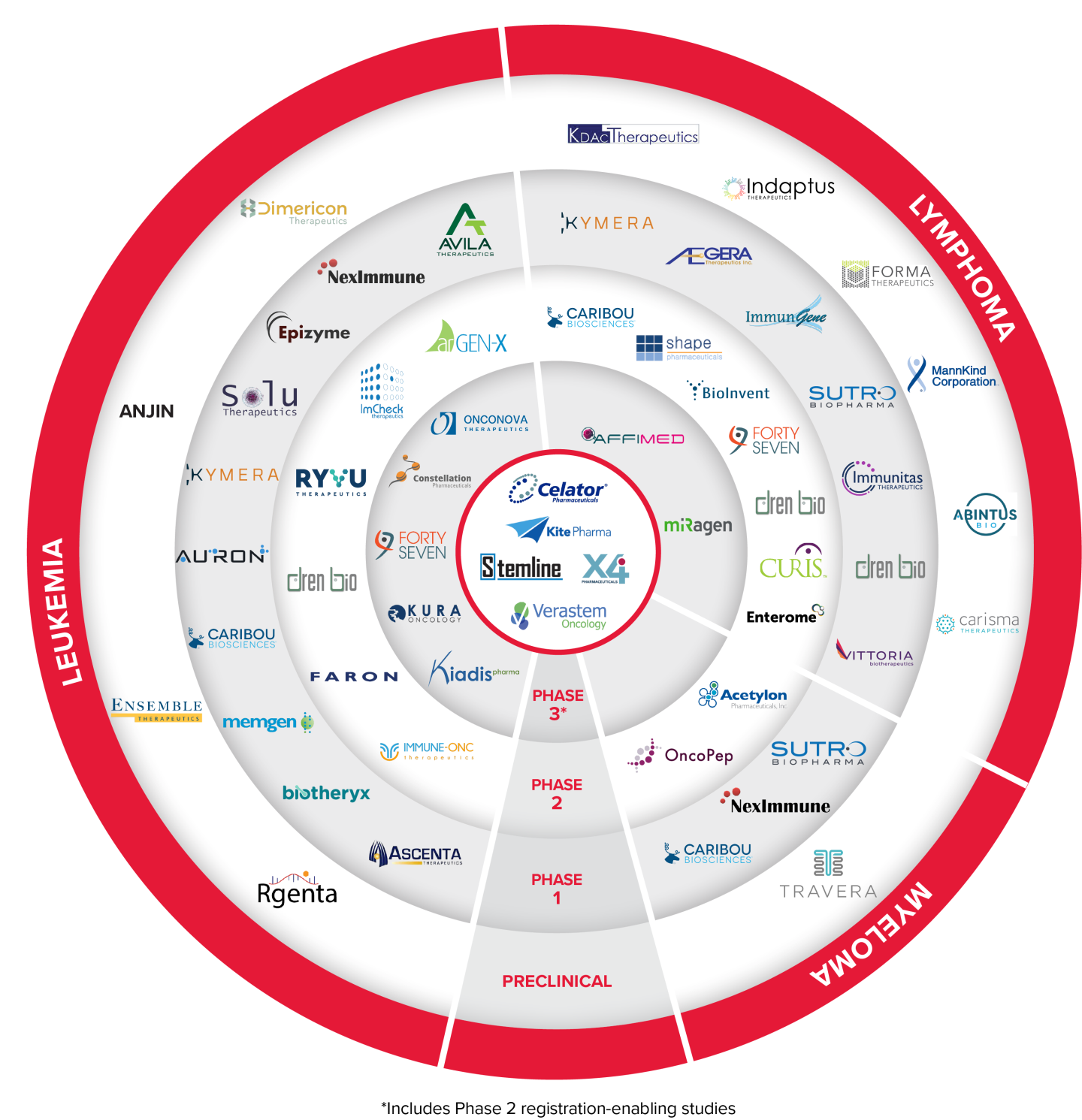
Select TAP Portfolio Assets in Company Sponsored Trials - Blood Cancer Development
TAP Partners
Auron Therapeutics
NEWEST PARTNER - JUNE 2025
Newtown, MA - Auron Therapeutics is a platform-powered company targeting cell-state plasticity to improve patient outcomes in oncology and inflammatory disease. Auron pioneered its AURIGIN platform, which uses AI and machine learning to compare cell states and identify novel drug targets, optimal development models, and biomarkers to facilitate proper patient selection, ultimately accelerating the development of effective and durable therapies.
AUTX-703 is a first-in-class, oral KAT2A/B degrader and is being evaluated in a Phase 1 dose escalation and dose optimization study in patients with relapsed/refractory AML or MDS (NCT06846606).
Affimed
Heidelberg, Germany - Affimed is a clinical-stage immuno-oncology company committed to giving patients back their innate ability to fight cancer by actualizing the untapped potential of the innate immune system using the proprietary ROCK® platform to enable a tumor-targeted approach to recognize and kill a range of hematologic and solid tumors.
AFM13 is bispecific tetravalent engager targeting CD30 on tumor cells and CD16A on NK cells and macrophages. AFM13 in combination with AB-101 (allogeneic natural killer cells) is currently in a Phase 2 clinical trial in relapsed or refractory Hodgkin lymphoma or CD30-positive PTCL (NCT05883449).
BioInvent International
Lund, Sweden - BioInvent International AB is a clinical-stage biotech company that discovers and develops novel and first-in-class immuno-modulatory antibodies for cancer therapy. The Company's validated, proprietary F.I.R.S.T™ technology platform identifies both targets and the antibodies that bind to them, generating many promising new drug candidates to fuel the Company's own clinical development pipeline and providing licensing and partnering opportunities.
BI-1206 is a novel anti-FcyRIIB antibody currently being studied in two Phase 1/2 trials, in combination with rituximab and acalabrutinib in indolent NHL (NCT03571568) and in combination with pembrolizumab in solid tumors.
BI-1808 is an anti-TNFR2 antibody being evaluated in a Phase 1/2a trial, as a single agent and in combination with pembrolizumab in patients with ovarian cancer, non-small cell lung cancer and cutaneous T-cell lymphoma (NCT04752826).
Caribou Biosciences
Berkeley, CA - Caribou is a clinical-stage biotechnology company, co-founded by CRISPR pioneer and Nobel Prize winner Jennifer Doudna, Ph.D., using next-generation CRISPR genome-editing technology to develop “off-the-shelf” (allogeneic) CAR therapies for hard-to-treat blood cancers.
CB-010, an allogeneic CD19 CAR-T cell therapy in which PD-1 was genetically disrupted in the CAR-T genome, leading to more durable anti-tumor activity in preclinical studies, is being evaluated in a Phase 2 clinical trial expansion cohort for second-line patients with large B cell lymphoma (NCT04637763).
CB-011, an allogeneic BCMA CAR-T cell therapy that is immunologically cloaked for enhanced persistence, is being evaluated in a Phase 1 clinical trial for patients with relapsed or refractory multiple myeloma (NCT05722418).
Constellation Pharmaceuticals
Munich, Germany - Constellation Pharmaceuticals was a clinical-stage biopharmaceutical company developing novel therapeutics that selectively modulate gene expression to address serious unmet medical needs in patients with cancer. MorphoSys acquired Constellation in July 2021 and more recently, Novartis acquired MorphoSys in July 2024.
Pelabresib (CPI-0610) is a small molecule inhibitor of bromodomain and extra-terminal (BET) proteins. Pelabresib in combination with ruxolitinib was studied in a Phase 3 clinical trial (NCT04603495) for myelofibrosis patients that have not been previously treated with Janus kinase inhibitors. Topline data and full details were presented during an oral session at the 2023 ASH Annual Meeting. All hallmarks of myelofibrosis were improved with the pelabresib and ruxolitinib combination versus placebo plus ruxolitinib, which is the standard of care in myelofibrosis.
Dimericon Therapeutics
Zurich, Switzerland - Dimericon is a private biotech company focused on exploring crosslinked helix dimers (Dimericons) as therapeutics and templates for small molecule development. Dimericon’s technology targets hard-to-drug intracellular protein-protein interactions using rationally designed mimetics of helix dimers. The Seed round of financing will support preclinical studies to further develop the current lead compound to be an IND ready clinical candidate in hematological malignancies.
Dren Bio
Foster City, CA - Dren Bio is a clinical-stage biopharmaceutical company focused on developing therapeutic antibodies for the treatment of cancer, autoimmune and other serious diseases. Dren Bio’s pipeline encompasses two distinct programs, the first focusing on the engineering of antibodies with enhanced antibody-dependent cellular cytotoxicity (“ADCC”) capabilities and the second revolving around its proprietary Targeted Myeloid Engager and Phagocytosis Platform.
DR-01 is a novel antibody targeting CD94 which is known to be upregulated on LGLL cells. DR-01 functions through depletion of target cells via ADCC by means of fratricide, a method in which the same cell type induces ADCC on each other. A Phase 1/2 trial is ongoing to assess the safety and efficacy of DR-01 in previously treated LGLL patients and cytotoxic lymphomas (NCT05475925).
DR-0201 is a first-in-class bispecific antibody capable of engaging tissue-resident and trafficking myeloid cells to induce deep B cell depletion via targeted phagocytosis. DR-0201 is currently being evaluated in a Phase 1 study in B-NHL patients (NCT06392477).
Enterome
Paris, France - Enterome is a clinical-stage biopharmaceutical company developing breakthrough immunomodulatory drugs for the treatment of cancer and immune diseases. Enterome’s pioneering approach to drug discovery is based on its unique and powerful bacterial Mimicry drug discovery platform, allowing it to analyze and uncover new biological insights from the millions of gut bacterial proteins in constant cross-talk with the human body. Its first-in-class small protein and peptide drug candidates modulate the immune system by closely mimicking the structure, effect or actions of specific antigens, hormones, or cytokines.
EO2463 is a clinical-stage off-the-shelf peptide-based immunotherapy and is currently enrolling in a multicenter, Phase 1/2 trial investigating EO2463 in monotherapy and in combination with standard of care - rituximab and rituximab in combination with lenalidomide – for treatment of patients with indolent NHL (NCT04669171).
Faron Pharmaceuticals
Turku, Finland - Faron Pharmaceuticals is a clinical stage biopharmaceutical company developing novel treatments for medical conditions with significant unmet needs caused by dysfunction of our immune system. The Company currently has a pipeline based on the receptors involved in regulation of immune response in oncology, organ damage and bone marrow regeneration.
Bexmarilimab, a novel anti-Clever-1 humanized antibody, is its investigative precision immunotherapy with the potential to provide permanent immune stimulation for difficult-to-treat cancers through targeting myeloid function. A Phase 2 study (BEXMAB) of bexmarilimab in combination with azacitidine is currently enrolling high-risk MDS patients in the US and Finland (NCT05428969).
ImCheck Therapeutics
Marseille, France - ImCheck Therapeutics is designing and developing a new generation of immunotherapeutic antibodies targeting butyrophilins, a novel super-family of immunomodulators.
ICT01 is a humanized, anti-BTN3A (also known as CD277) monoclonal antibody that selectively activates γ9δ2 T cells, which are part of the innate immune system that is responsible for immunosurveillance of malignancy and infections. A clinical trial (EVICTION) is currently enrolling a Phase 2 cohort expansion of ICT01 in combination with azacitidine and venetoclax in patients with untreated acute myeloid leukemia (NCT04243499).
Immune-Onc Therapeutics
Palo Alto, CA - Immune-Onc is a clinical-stage cancer immunotherapy company dedicated to the discovery and development of novel myeloid checkpoint inhibitors for cancer patients.
IO-202 is a first-in-class monoclonal antibody that antagonizes LILRB4. IO-202 is in a Phase 1 expansion clinical trial in combination with azacitidine for the treatment of advanced monocytic AML and CMML (NCT0437243). In June 2023, the FDA granted Fast Track designation to IO-202 for the treatment of R/R CMML, which follows the R/R AML Fast Track designation received in February 2022.
Immunitas Therapeutics
Waltham, MA - Immunitas is committed to discovering and developing novel, differentiated therapeutics for patients with cancer using a discovery engine that combines deep expertise in single-cell genomics with customized machine learning approaches to elucidate immune cell populations that are key actors in immuno-oncology.
IMT-009 is a first-in-class NK and T cell modulator targeting CD161 that is being developed for the treatment of solid tumors and lymphomas and is in a Phase 1 clinical trial (NCT05565417).
Kura Oncology
San Diego, CA - Kura Oncology is a clinical-stage biopharmaceutical company committed to realizing the promise of precision medicines for the treatment of cancer with a pipeline that consists of small molecule drug candidates that target cancer signaling pathways.
Ziftomenib (KO-539) is selective small molecule inhibitor of menin. Ziftomenib is currently in a Phase 2 registration-directed clinical trial in patients with NPM1-mutant relapsed or refractory AML (NCT04067336) completed enrollment in May 2024, with topline data presented in early 2025.
The FDA has accepted and granted Priority Review of the NDA for ziftomenib in adults with R/R NPM1-mutant AML. The PDUFA target action date is November 30, 2025.
Rgenta Therapeutics
Cambridge, MA - Rgenta Therapeutics is developing a pipeline of oral, small-molecule RNA-targeting medicines with an initial focus on oncology and neurological disorders. Rgenta has a proprietary platform to mine the massive genomics data to identify targetable RNA processing events and to design small-molecule glues to modulate the interactions among the spliceosome, regulatory proteins, and RNAs.
Rgenta is working closely with LLS TAP to further develop RNA-targeting molecules by supporting preclinical studies with the goal of moving towards clinical development in hematological malignancies.
Ryvu Therapeutics
Krakow, Poland - Ryvu Therapeutics is a clinical-stage drug discovery and development company focusing on novel small molecule therapies that address emerging targets in oncology using a proprietary discovery engine platform.
RVU120 is a highly selective first-in-class CDK8/CDK19 small molecule inhibitor. Ryvu is currently enrolling several Phase 2 clinical trials: RVU120 in combination with venetoclax for patients with relapsed/refractory AML (RIVER-81, NCT06191263), as monotherapy for patients with low-risk myelodysplastic syndromes (REMARK, NCT06243458) and RVU120 as monotherapy and in combination with ruxolitinib for patients with myelofibrosis (POTAMI-61, NCT06397313).
Solu Therapeutics
Boston, MA - Solu Therapeutics is a biotechnology company dedicated to developing next-generation therapeutics to eliminate disease-driving cells in cancer, immunology and other therapeutic areas. The company’s proprietary CyTAC (Cytotoxicity Targeting Chimera) and TicTAC (Therapeutic Index Control Targeting Chimera) platforms enable the development of innovative medicines that combine the target-binding capability of small molecules with the therapeutic power of biologics.
STX-0712 is a CyTAC targeting the GPCR CCR2, which is a selective marker expressed at high levels on pathogenic monocytes, key drivers in some hematological malignancies, is being evaluated in a Phase 1 clinical trial for patients with CMML. (NCT06950034).
Vittoria Biotherapeutics
Philadelphia, PA - Vittoria Biotherapeutics is developing novel CAR-T cell therapies that transcend the limitations of current cell therapies. Based on technology exclusively licensed from the University of Pennsylvania, Vittoria's proprietary Senza5 platform unlocks the antitumor potential of engineered T cells and utilizes a five-day manufacturing process to maximize stemness, durability, and target cell cytotoxicity. By acting on the fundamental biology of T cells, Senza5 can be used to improve the efficacy of engineered T cell therapies with pipeline applications in oncology and autoimmune diseases.
VIPER-101 is an autologous, dual population CD5-knockout CAR-T cell therapy for T-cell Lymphoma featuring the novel Senza5 platform technology. The Phase 1 dose escalation clinical trial is currently enrolling patients (NCT06420089). Several other products are in earlier preclinical development.
















