TAP accepts and reviews partnering inquiries year round!
TAP collaborates with and makes equity investments in biotech companies to support the development of novel platforms, first-in-class assets addressing high unmet medical needs, emerging patient populations and orphan indications.
- Click here to download the initial inquiry form
Send completed inquiry form & non-confidential presentation to Dr. Blaine Robinson, Vice President, Therapy Acceleration Program®.
Want to know more about applying to TAP - download a presentation and a one-pager for more information.
Listen to a network of experts share their insights and experience on TAP.
Interested in learning how a donation can help support & impact TAP?
Through TAP, investment-savvy donors provide opportunity funding to enable LLS to make equity investments in biotech companies with novel therapeutic projects—with the potential for a significant return back to LLS. This return comes in many forms: in advances in the field, improved therapies, new standards of care and innovative technologies that transform the entire landscape of medical science and cancer therapeutics. Returns are also seen in the ability of TAP biotech partners to raise additional funds to advance new therapies towards approvals, and in the financial returns that come back to LLS to re-invest in TAP.
For more information on how to support TAP, please contact Sheena Mehta, Director of Philanthropy, TAP ([email protected]).
Download our Impact Investing Brochure for additional information.
How did TAP impact the development of Elzonris?
LLS awarded several grants to Dr. Arthur Frankel between 1998-2008 to develop fusion protein therapies for blood cancers, including SL-401 (now known as Elzonris). TAP then partnered with Stemline Therapeutics in 2013 to support key clinical studies for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Listen to what Dr. Arthur Frankel and Peter MacDonald, SVP of Corporate & Business Development of Stemline Therapeutics, have to say about LLS, the role TAP played in the development of Elzonris and the value TAP created for Stemline beyond the funding amount.
Elzonris® is the first approved therapy for blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare blood cancer.
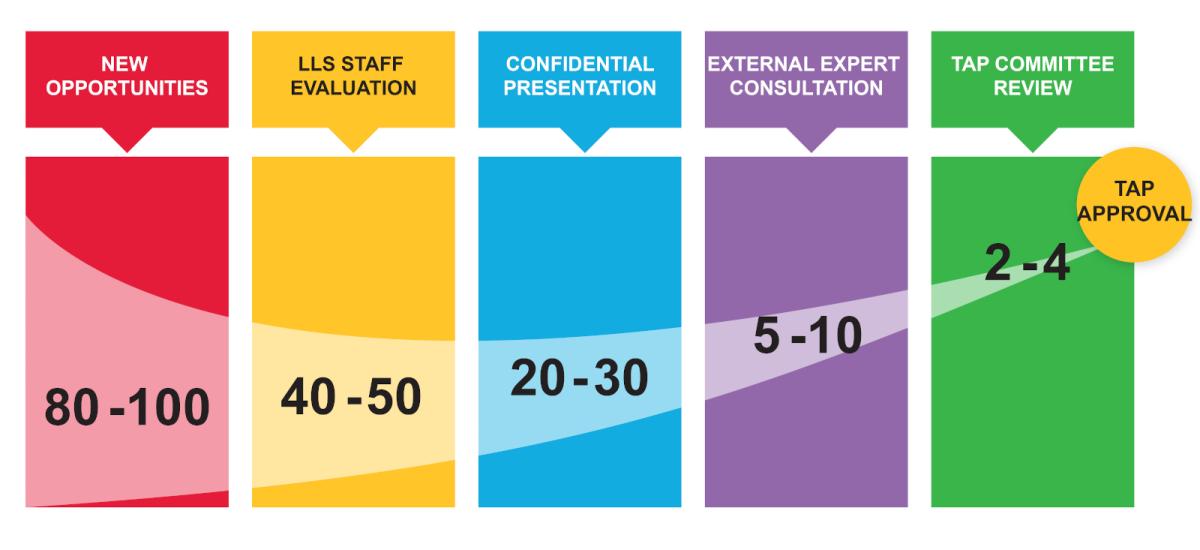
Due Diligence Process
The TAP team applies industry expertise and a rigorous due diligence to identify companies that are best positioned to advance novel research and therapeutic technologies in areas with the greatest potential impact on patients.
Every year, TAP reviews opportunities from approximately 80-100 biotech companies and only invests in several new projects annually, with an open partnering cycle all year long.
Diligence typically takes 3-6 months, but varies based on company responsiveness to LLS requests.