Search Results
Follow-Up Care
Find more information about follow-up care, including what to expect, long-term and late effects of treatment, survivorship clinics, and other resources, such as The National Comprehensive Cancer Network (NCCN) treatment guidelines.

Bill
My name is Bill Bannon. I am a semi-retired child support magistrate for the Minnesota State Courts. In April 2017 I experienced shortness of breath, a sore-throat and bleeding gums. I was diagnosed with acute myeloid leukemia (AML), was stabilized, and started on intensive chemotherapy. It was soon learned that I had a FLT-3 mutation of this cancer, the most serious and difficult to treat. The only possibility for any chance at survival was a bone marrow or umbilical stem cell transplant (BMT). I chose to continue my treatment at the University of Minnesota.

Zeke
On April 16, 2022, I brought my son Ezekiel "Zeke" to a sick appointment because of leg pain, stomach pain, and a fever over the last few days. We were sent for some additional testing (bloodwork and an X-ray of his stomach area). Later that day, we received a call from the doctor saying we needed to get Zeke back to the hospital fast because his bloodwork wasn't normal, and he had an enlarged liver and spleen. We woke up Easter morning in the hospital for the first of what would become over 100 nights at the hospital throughout his treatment.

Tak Wah Mak, Ph.D.
A Leading Scientist Studying Precision Medicine Approaches for Leukemia and Lymphoma
Dr. Tak Wah Mak is one of the world’s most cited and accomplished scientists. After earning his PhD, Mak was recruited by the Ontario Cancer Institute (now Princess Margaret Cancer Centre) in Toronto, Canada for a postdoctoral fellowship.

GiGi
When I was diagnosed in 2002 I was in such a fog, all that I can really remember is the doctor saying "You have..." I had acute myeloid leukemia and given 6 weeks to live. I just felt that could not be the end for me.
I went through the chemo, hospital stays all while trying to raise 4 small children and I made it through it all. Thank goodness, right? Fast forward to 2017 the day after my 48th birthday. I began to feel weak and extremely fatigued, without trying to "self- diagnose" myself, this feeling was all too familiar.

Aleta
I was diagnosed on August 13, 2013 with T-cell acute lymphoblastic leukemia. It was totally out of the blue. I had been married for just a year at the time of my diagnosis and sadly my husband couldn't handle a wife with cancer and we divorced.
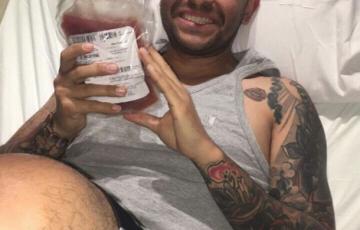
Andre
In July 1993 I was blessed with my son, Andre Christopher Jordan. He's my heart, pride and joy. Throughout his young life, Andre's has humbled me with his love, kindness, strength, loyalty and faith. Two years ago, he and his fiancé, Ana were blessed with their son. He was overjoyed with being a new father and had marriage plans in the works. He had just received a promotion at work that he had worked hard for and all was well. Then suddenly, Andre's life suddenly took a tragic turn.

Jayson
On October 29, 2023, I was diagnosed with acute myeloid leukemia (AML) at the age of 24. Before cancer, I moved through life without a care in the world, living each day like tomorrow was guaranteed. That all changed in an instant.

Pilar
My name is Pilar, and I am 34 years old. I used to work for the workforce board of Philadelphia, working with youth, until I was diagnosed with acute myeloid leukemia (AML) back in October 2020. It has been a hard battle, but I am positive and blessed to have the support of my family and friends. Additionally, I was very fortunate my sister, Marcela, was a match to be my donor.

Rose
My brother Hopoate is a leukemia survivor. At the young age of 2, he was diagnosed with acute lymphocytic leukemia (ALL) and finished his chemotherapy when he was 4-1/2 years old. He has been cancer-free for about 17 years now. He is now 21 and thriving.

Anna
As many of you know, in 2015 our daughter Anna was diagnosed with acute lymphoblastic leukemia (ALL). She had a very successful treatment, and God answered my wife and my prayers. As of March 2023, Anna has been cancer-free for five years making her a cancer survivor. On October 21, Aimie, Anna, and I will participate in the Light The Night (LTN) walk for The Leukemia & Lymphoma Society (LLS). Funds raised through LTN allow LLS to fund treatments for patients who have blood cancer. As you can imagine, this cause is very special to Aimie and me.

Wendell
Wendell Ison was diagnosed with chronic lymphocytic leukemia (CLL) on June 6, 2006. It was through his own battle that he decided to create the team known as "Wendell's Warriors" as a way to give back and make a difference in people's lives by raising money for the Leukemia Lymphoma Society (LLS).
Deana
In May 2008 at nine years-old, I was diagnosed with acute lymphoblastic leukemia (ALL). I went from being told I had a 10 day stomach virus to being told I had leukemia. The doctors said told I didn't come in when I did, I could of lost my life.
A year went by, and I was stuck in the hospital missing out on family and important events. I even had my tenth birthday in the hospital, but I was too sick to enjoy it.

Joey
Joey Renick is a three-time acute lymphoblastic leukemia (ALL) survivor. He was first diagnosed at the age of 3, then 18, and again at 22. Joey has received years of chemotherapy, radiation, and a bone marrow transplant. Since receiving his bone marrow transplant in June 2016, Joey has married his wife Caylee, completed nursing school, began and continues to work as a bone marrow transplant nurse, and will be a dad soon.

Sydney
My grandfather, Michael, passed away from leukemia in 1991. While I never had the pleasure of meeting him, I have always considered him to be my guardian angel. My grandmother and best friend, Patrice, has been a loyal advocate, supporter, and donor to The Leukemia & Lymphoma Society (LLS) ever since his passing. Additionally, she served as vice president for two years and president of LLS for four years. We love how committed LLS is to the research and care of those with blood cancer.

Geoff
I was diagnosed with acute myeloid leukemia (AML) on April 13, 1995, at Arlington Hospital (now VHC Health) and was hospitalized there for 32 days for chemotherapy. I received a second round of chemotherapy (high-dose) at Memorial Sloan-Kettering Hospital a few weeks later. Afterward, I received an autologous stem cell transplant at Sloan Kettering. I was discharged on August 13, 1995, and readmitted in late August for two days for an infection. I have been cancer-free since then.

Julian
I was a 10-year-old kid when I was diagnosed with leukemia. I was a happy, energetic kid who loved being outside and was a straight-A student. However, my whole childhood was put on hold indefinitely once I had cancer. Rather than having sleepovers at my friend’s houses, I was sleeping over at the hospital. It was hard to lose my energy, smile, confidence, and hair as treatment took over my life. It was really hard to understand as I was just a kid getting injections, procedures, chemotherapy, and surgery without truly understanding why.
Follow-Up Care
Click here for information about follow-up care, including what to expect, long-term and late effects of treatment, survivorship clinics, and other resources such as The National Comprehensive Cancer Network (NCCN) treatment guidelines.
Use the Survivorship Workbook to collect all the important information you need throughout diagnosis, treatment, follow-up care and long-term management of a blood cancer.
Treatment Outcomes
In some people with PV, the disease remains stable for many years. In many people, life expectancy is the same as it would be if they did not have PV. With careful medical supervision and therapy, PV can usually be managed effectively for a long time. In some cases, however, it may progress to another type of blood disease, such as myelofibrosis or acute myeloid leukemia.
Treatment Outcomes
A few decades ago, there were very low cure rates in both children and adults diagnosed with ALL. Today, childhood ALL has one of the highest cure rates of all childhood cancers, approaching 92 percent for children younger than 15 years and more than 94 percent for children younger than 5 years.

Sarah
I am the mother of Madelynn, aka Maddie, who was diagnosed with biphenotypic acute leukemia (BAL) on March 13, 2023, following months of illness initially thought to be a sinus infection. Despite multiple doctor visits and two trips to the emergency room, it wasn’t until Maddie’s condition worsened that she received the correct diagnosis, which involved both acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) with the Philadelphia chromosome. Maddie immediately began an aggressive chemotherapy regimen and spent the first month of her treatment in the hospital.

Sue
It’s 2015 and I am doing my happy dance! It’s been 20 years since my bone marrow transplant for my chronic myeloid leukemia (CML) and I am still here enjoying what life brings my way.

George
My awareness of The Leukemia & Lymphoma Society (LLS) began years ago when a close family member was diagnosed with leukemia. In the wake of such devastating news, I felt compelled to lend a hand. I am honored to join the LLS family in their fight to cure blood cancers.

Nanci
I was diagnosed with chronic myelogenous leukemia (CML) in January 2009. My doctor said the average life span was three years if not for the newer drug called Gleevec, a drug that The Leukemia & Lymphoma Society (LLS) had been involved in the research of it. I started on Gleevec in February, but after a week I was taken off it to bring my immune system back up. Back on Gleevec, I reached remission in July 2009. I now have been in remission for 12½ years. I now take the generic brand due to the cost.

Kaidyn
Kaidyn was only six months old when he was diagnosed with acute myeloid leukemia (AML). About two months later, he received a bone marrow aspiration and was started on chemotherapy. Over the next ten months, Kaidyn was in and out of Children's Hospital of Oakland. It was there that he took his first steps, said his first words, and even flirted with every nurse in the oncology unit! Kaidyn is now a healthy three-year-old boy with an ear-to-ear grin who participates in his local Light The Night Walk each fall.