The more we learn more about the underlying mutations that drive cancer the better we get at more precisely hitting those targets with specialized, less toxic treatments. We now understand that a one-size-fits all approach to treating cancer is ineffective for many patients. As The 62nd American Society of Hematology (ASH) annual Meeting ends, let’s take a look at results from studies of targeted therapies that work by interfering with the altered genes that cause cancer cells to grow and spread. Much of the work stems from research supported by LLS.
Precision Medicine in Acute Myeloid Leukemia
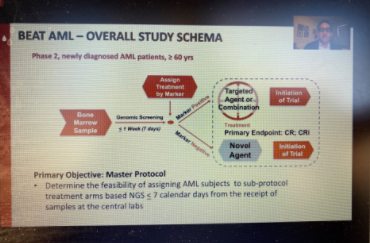
More than four years ago, LLS launched its Beat AML Master Clinical Trial, a precision medicine study that uses genomic technology to identify a patient’s subtype of acute myeloid leukemia (AML) and matches them with a targeted therapy in one of the Beat AML sub-studies. Eytan Stein, MD, of Memorial Sloan Kettering Cancer Center, presented data from one of those sub-studies on Monday showing that enasidenib is a highly effective drug in newly diagnosed older AML patients with the IDH2 mutation. IDH1 and IDH2 mutations make up about 20 percent of AML patients. Stein said that 47 percent of the patients in the study had a complete response to enasidenib, and the median overall survival was 24.4 months (half the patients were still alive after more than two years).
Patients who did not respond to enasidenib alone were given the drug combined with another drug, azacitidine, whichswitches on a tumor-suppressing gene. Seven out of 17, or 41 percent, of those patients achieved a complete response. Dr. Stein said Beat AML will test enasidenib with other novel agents in patients who are resistant to the drug on its own. The FDA approved enasidenib in 2017 for IDH2-positive AML patients who have relapsed or don’t respond to previous treatment; it is being tested in Beat AML as a first-line treatment for newly diagnosed patients. A recent publication about the Beat AML study in Nature Medicine showed that patients in the study generally did better than those who opted for standard treatment.
A Reformulated Standard Therapy Continues to Improve Outcomes
In 2009, LLS begin supporting a clinical trial of a drug called CPX-351 through its Therapy Acceleration Program (TAP), a strategic venture philanthropy funding initiative. LLS partnered with Celator Pharmaceuticals, which was subsequently acquired by Jazz Pharmaceuticals, to advance CPX-351, an innovative formulation of two existing chemotherapy drugs used to treat AML. The FDA approved CPX-351 in 2017 for AML patients with high-risk (secondary) AML, caused by previous cancer therapy, or that evolves from another disease such as myelodysplastic syndromes (MDS). A study presented on Monday shows that five years out, CPX-351 continues to outperform standard chemotherapy, with patients experiencing deeper, more enduring responses.
A Game-Changer for Multiple Blood Cancers: Venetoclax
More than two decades ago, LLS began supporting research of BCL-2, a protein found to prevent an important biological function called apoptosis – a process that rids the body of damaged cells. The overexpression of BCL-2 allows the proliferation of cancer cells. The resulting drug, venetoclax, has proven to be a game-changer for both chronic lymphocytic leukemia (CLL) and AML. Multiple presentations at the ASH meeting showed impactful results of venetoclax in various combinations with other therapies for both of these blood cancers.
For example, LLS-funded researcher Dan Pollyea, MD, MS, of University of Colorado, showed that venetoclax combined with azacitidine, was far more effective than azacitidine alone, for AML patients with IDH1 and IDH2 mutations who are too old or sick to withstand standard chemotherapy. Findings were eye-popping: between 72-78 percent of patients treated with the combination responded, as compared to only 7-10 percent who got azacitidine with just a placebo. Median overall survival was 24.5 months for the combination vs. 6.2 months for the azacitidine and placebo (median overall survival is the length of time from the start of treatment that half of the patients are still alive).
Venetoclax’s impact was clear in multiple CLL trials as well, with study after study showing that chemo-free combinations involving venetoclax and other targeted therapies, such as ibrutinib and rituximab, continue to crush the cancer. Many patients remain completely free of cancer cells, even when using the most sophisticated, sensitive tests to search for residual disease. Many of the remissions appear to be long-lasting.
Ibrutinib and related drugs block a protein called BTK (Bruton’s tyrosine kinase), present in several types of blood cancers. LLS has provided significant long-term investment in BTK inhibitors, which are highly effective in controlling the disease, particularly when combined with other therapies like venetoclax or rituximab, an antibody therapy. But some patients are resistant to BTK inhibitors.
At the ASH meeting, an investigative therapy called LOXO-305 showed very promising results for such patients. LOXO-305 is also a BTK inhibitor but designed in such a way to overcome resistance to the earlier versions. In one study, 88 of 121 patients with CLL or small lymphocytic lymphoma had their tumors shrink at least 30 percent, a response rate of 62 percent when treated with the drug. The response increased to 84 percent in patients followed for 10 or more months.
More Progress for LLS TAP Partners
I previously discussed results presented by our TAP partners Celator (see above) and Kite Pharma. Other TAP partners also shared impressive results.
Myelofibrosis (MF) is a disorder in which abnormal bone marrow stem cells produce scar tissue that replaces healthy marrow and patients experience enlarged spleens and anemia.
TAP partner, Constellation Pharmaceuticals, showed that its drug CPI-0610 alone, and in combination with another therapy, ruxolitinib, improved symptoms of MF patients. Ruxolitinib is a targeted therapy that inhibits a mutation called JAK2, commonly found in myeloproliferative neoplasms (MPN), a family of blood cancers that includes MF. CPI-0610 targets a family of proteins called bromodomain and extra-terminal motif (BET) also common in MF. In a study of newly diagnosed MF patients, 42 of 63 patients (67 percent) achieved at least a 35 percent reduction in spleen volume at 24 weeks, the primary endpoint for the trial. Seven of 23 (30 percent) of patients who received CPI-0610 as a monotherapy responded with at least a 35 percent reduction in spleen volume.
New treatments for patients with a rare and lethal subtype of leukemia
LLS first began supporting research by Jolanta Grembecka, PhD, at University of Michigan in 2009, first through our academic grants program, and later through TAP. Her work is focused on a leukemia subtype associated with abnormalities in the mixed lineage leukemia (MLL) gene. When another protein called menin interacts with the MLL fusion proteins, the result is MLL leukemias, which occur both in children and adults. Grembecka's team developed a first-in-class small molecule compound that inhibits the menin-MLL interaction. In 2014, Kura Oncology licensed the drug from Grembeca’s lab. Kura presented very early results from the first-in-human study of this drug, KO-539, in patients with relapsed or refractory MLL acute myeloid leukemia. While the study was small, it looks promising with several of the patients responding and achieving remissions lasting at least several months. We’ll be keeping an eye on the progress of this therapy.